Introduction
In the case of hearing aids, customers are concerned not just about design or functional difference but about reliability—whether or not a product can be relied on to deliver clear, natural sound predictably. Producers’ secret is quality control.
ELHearing, as a high-quality hearing aid manufacturer, has always valued quality control as its lifeblood. On raw material selection, shipping of finished products, we have established a sophisticated process control system involving R&D, production, testing, and packaging. In this article, readers will gain an inside picture about how ELHearing maintains each hearing aid in the best performance and stability due to its rigorous and scientific quality control process.
1. Source Control: Raw Material and Supply Chain Quality Control
The world’s finest hearing aids are matched by the world’s finest raw materials. ELHearing implements strict quality control in supply chain management by undertaking several audits of each critical component.
Our primary materials are microphones, speakers, DSP chips, battery modules, and plastic casings. Each component undergoes the following procedures:
-
Supplier Certification and Qualification: New suppliers must be certified ISO and CE and provide complete material test reports before being included in our partnership list.
-
Incoming Quality Inspection: Materials undergo sampling inspection before warehousing, such as dimensional tolerance, impedance testing, noise figure testing, and temperature testing.
-
Batch Traceability: Every batch of materials is individually numbered so that traceability can be achieved in the entire production process and any problem can be identified promptly.
Statistics indicate that owing to our stringent Quality Control system, ELHearing’s raw material qualification rate is always above 99.6%, providing a good foundation for the subsequent production stability.
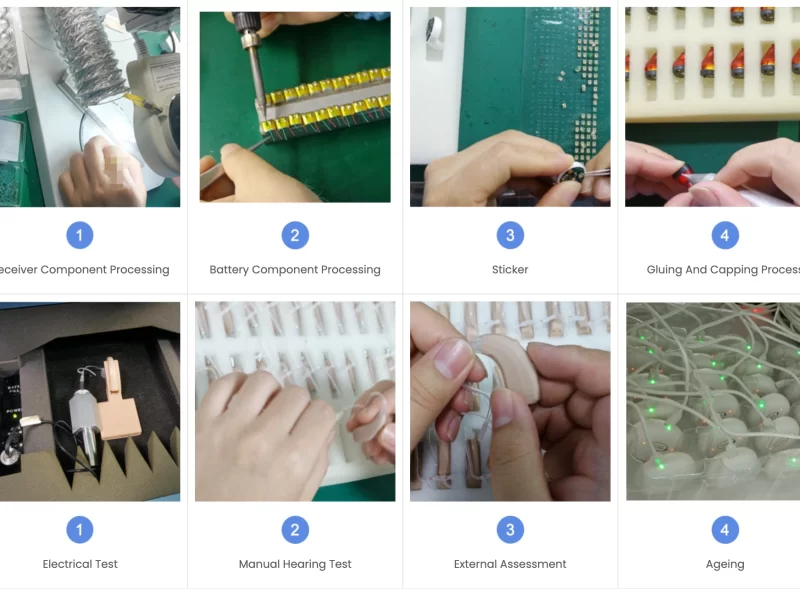
II. Precision Production: Quality Control Nodes in the Manufacturing Process
In the course of producing hearing aids, micron-level changes can distort sound or result in signal interference. ELHearing has built over 15 nodes of quality control into its production process. From assembly to welding, from injection molding to acoustic calibration, the whole process is done through both automated quality checking tools as well as manual inspection.
For example, during the PCB soldering process, we check solder joints with a 99.9% quality rate using a fully automatic optical inspection (AOI) system. During acoustic tuning, hearing aids are subjected to testing inside an acoustic chamber to ensure that the frequency response curve is not more than ±1.5dB from the standard curve.
In addition, the production line boasts a “first article inspection” system where the entire production is inspected after every change in material or mold to prevent systematic deviations.
This exhaustive quality control process not only ensures consistency of products but also reduces rework to a minimum while maintaining ELHearing’s production qualification rate above 98%.
III. Performance Testing: Testing Each Device to Its Limits
Hearing aids are medical-quality electronic devices, and their stability of performance relies directly on the results of hearing rehabilitation by the user. ELHearing has an in-house product performance laboratory which conducts strenuous quality control testing of every single product.
Principal items to be tested are:
-
Acoustic Performance Testing: Including total harmonic distortion (THD), equivalent input noise (EIN), maximum sound output (MPO), and other specifications.
-
Electrical Stability Testing: Through temperature cycling and high humidity testing, it simulates product performance in different climatic conditions.
-
Durability Testing: Like switch life testing (≥100,000 cycles) and battery compartment opening and closing multiple times testing.
-
Drop and Vibration Testing: It ensures hearing aids also function well even if they are dropped accidentally.
Under these rigorous quality control procedures, ELHearing products receive a mean of over 30 performance inspections prior to leaving the factory. Figures show that our mean rate of failure is under 0.3%, significantly lower than that of the industry (around 1.2%).
IV. Certification and Standards: Ensuring International Credibility in Quality Control
ELHearing’s quality control efforts extend beyond internal monitoring and adhere strictly to international standards of quality. We hold ISO 13485 certification in medical device quality management systems and CE and FDA registrations, which attest that our manufacturing processes fully satisfy global medical device standards.
In addition, we are annually audited by independent third-party entities (such as SGS or TÜV) to ensure ongoing effectiveness of our quality control systems.
Our internal audit function also regularly conducts spot checks and optimizes production processes to ensure consistent adherence to quality levels.
These international certifications not only reinforce our brand reputation but also give confidence to foreign partners. To international distributors, choosing ELHearing means choosing a sound, trusted, and certified business partner.
V. Continuous Improvement: Data-Driven Optimization of Quality Control
In ELHearing, we believe quality control is not a fixed mechanism but an evolving process. According to our digital management system, we can monitor production data in real time and integrate quality indicators with equipment running data to achieve intelligent early warning and traceability.
For example, when there are irregular fluctuations in a manufacturing line, the system will automatically display a report and notify engineers for examination. With the analysis of big data, we are able to detect potential defect patterns and proactively improve design or material selection.
ELHearing has also established a “closed-loop quality feedback system” where customer feedback collected from after-sales service directly flows into our quality database and is utilized to promote product design and manufacturing processes.
This data-driven quality control system enables us to continuously improve product reliability, making paradigm shifts in stability and durability with every generation of hearing aids.
Conclusion
Quality control is an integral component of product stability and reliability in manufacturing medical hearing devices. ELHearing has established a systematic quality assurance system through a comprehensive quality management process, from acquiring raw materials to production testing, performance verification, and continuous improvement.
This science-based and data-driven management style not only ensures that our hearing aids reach international standards but also win customers’ trust worldwide. In the future, we will continue to improve our quality control system, so each user can “hear the world with peace of mind.”
About ELHearing
ELHearing is a professional hearing aid manufacturer with experience in researching, designing, manufacturing, and exporting high-performance digital hearing aids. We possess a sophisticated quality control system and overseas qualifications such as ISO13485, CE, and FDA, ensuring our products meet medical-grade security standards.
We offer OEM/ODM customization and wholesale business, and our product categories include BTE, ITE, and RIC models, which are widely applied in hearing centers, medical outlets, and brand distribution channels.
With over a decade of industry experience and sound technical support, ELHearing is now a trusted partner to clients in over 30 countries worldwide. ELHearing is a trustworthy hearing aid manufacturer that offers higher quality control and a professional team to guide you to high-quality solutions.
